Researchers from the University of Virginia's School of Engineering and Applied Science have developed a promising drug-carrying molecule that could revolutionize treatment for chronic and severe respiratory diseases. The project, led by Assistant Professor Liheng Cai and his team—including Ph.D. students Baiqiang Huang and Zhi-Jian He—focuses on a new nanocarrier designed to bypass the lung's natural defenses. Their work, detailed in a study published in ACS Nano, uses an innovative device called a "micro-human airway," which mimics the structure and function of human airways.
Overcoming Lung Defenses
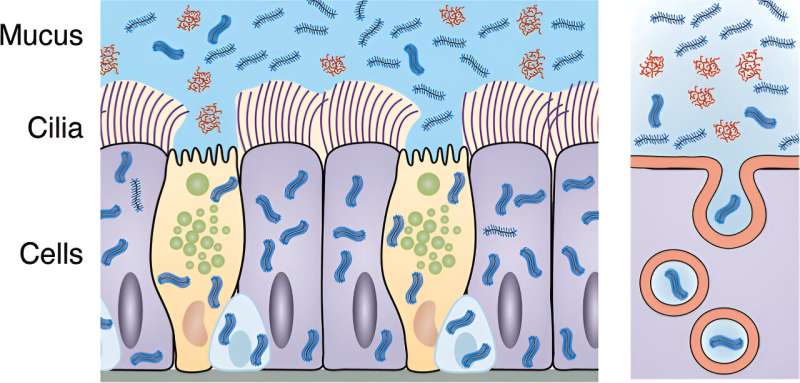
The lungs have built-in defenses to trap and expel pathogens or particles. While this system protects against illness, it also makes it challenging for medications to reach their intended targets. According to Huang, this barrier complicates the treatment of diseases like asthma, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis.
The new polymer, named bottlebrush polyethylene glycol (PEG-BB), is designed to navigate these barriers effectively. It mimics mucins, natural glycoproteins that help form mucus. The bottlebrush shape—characterized by a central backbone with branching "bristles"—allows PEG-BB to move through the dense mucus and gel layers that surround cilia (tiny hair-like structures on cell surfaces).
To test its effectiveness, the researchers used their micro-human airway device to culture human airway epithelial cells. They introduced fluorescent PEG-BB molecules into these cells from two directions and used dyes to highlight the mucus and periciliary layers surrounding the cilia. This setup allowed them to observe how well the PEG-BB molecules traversed these layers.
Advancements and Future Goals
Huang described the micro-human airway as a precise environment for studying lung defenses without harming living subjects. Cai's lab, known for its cutting-edge research in bottlebrush polymers, is also working on other innovations, such as 3D bioprinting techniques for organ printing.
The team believes that PEG-BB could significantly improve treatments for lung diseases with fewer side effects and might also be applicable to other conditions affecting mucosal surfaces. Their next step involves testing PEG-BB's ability to deliver drug molecules across mucus barriers using both in vitro and in vivo models in mice.

:max_bytes(150000):strip_icc():format(webp)/alec-baldwin-torino-film-festival-121824-3cf7cfbe5c4f4bcbb9197de4de062ea6.jpg?strip=all&resize=370,370)
:max_bytes(150000):strip_icc():format(webp)/Jimmy-Fallon-and-Prince-Harry-092624-baa1362d743d4f39a7d60bf57eb6e8b2.jpg?strip=all&resize=370,370)
:max_bytes(150000):strip_icc():format(webp)/randy-moss-121324-40b858c71b0f40bfa399e2fe00152b2a.jpg?strip=all&resize=370,370)
:max_bytes(150000):strip_icc():format(webp)/scandal-kerry-washington-121824-c51a2b5f0ef741268474bec2498c285a.jpg?strip=all&resize=370,370)
